Determine the Number of Significant Figures in the Following Measurements:
Estimate the number of significant digits from the following computations. Perform the following calculations and express the answer in the correct units ad number of significant figures.
Precision Accuracy Measurement And Significant Figures Persuasive Essay Outline Educational Worksheets Education Templates
In this problem we have to determine the number off significant.

. Test your ability to find how many significant figures are in a number. Rules for significant figures. Determine the number of significant figures in each of the following measurements.
Express its area with the proper number of significant figures in the specified unit. The following measurement. All non-zero numbers are always significant.
For example 400 has three significant figures. Zeros within a number are always significant. All non-zero numbers are significant.
Refer to the Appendix or your text to review significant figures. Digits from 1 to 9 are always significant and have infinite number of significant figures. Determine the number of significant figures in each of the following.
684 624 and 854 all have three significant figures. M g m_g m g. Complete each of the following calculations and round your answer to the correct number of signifi- cant figures.
Enter whole numbers real numbers scientific notation or e notation. 150 L 3 4 L Answer. 21 times 109 g d.
What is the number of Significant Figures in each of the following measurementsa 4867 mib 56 mLc 60104 tond 2900 ge 402 gcm3f 00000003 cmg. 653280 5 For what angle of a projectile is its range equal to zero. 5546 g 289 g Answer.
This problem has been solved. Physics questions and answers. 11 soccer players b.
You can use this calculator for significant figures practice. XDetermine the number of significant figures in the following measurement. 17 Determine the number of significant figures in the following measurements.
Calculate the following using the correct significant figures 1. University Physics Volume 1 0th Edition Edit edition. 52 x 10 3 x 6732 x 10 3 Write 45378212 correct to 3 significant digitsfigures.
A 0 or 30 b 0 or 45 c 90 or 0 d 90 or 45. 312 kPa - 00035 kPa. Chemistry questions and answers.
Solutions for Chapter 1 Problem 78P. Determine the number of significant figures for this height measurement. A 00009 b 154500 c 6103 d 87990 and e 3042.
A 4867 mi b 56 mL c 60104 tons d 2900 g e 402 gcm 3 f. 015 cm 115 cm 2051 cm Answer. Determine the number of significant in each of the following measurements.
2051 mL Convert the following numbers into scientific notation. A 4867 mathrmmi b 56 mathrmmL c 60104 tons d 2900 mathrmg e 402 mathrmg mathrmcm3 f 00000003 mathrmcmmathrmg 07 mathrmmin h 46 times 1019 atoms. Determine the number of significant figures in each of the following measurements.
1563300 x 10¹¹ m. 135 m x 2467 m Answer. Zeros that do nothing but set the decimal point are not significant.
Measurements must always be reported to the correct number of significant figures because calculated answers often depend the numbers of significant figures in the valued used in the calculation. Example inputs are 3500 350056 35 x 103 and 35e3. SOLVEDDetermine the number of significant figures in each of the following measurements.
0021 cm x 32 cm x 1001 cm Answer. Solve the following 476 562 3321 and find the number of significant digitsfigures. Count how many significant figures are in a number and find which digits are significant.
A 11 mL b 4901 g C 00510 m3 d 91 x 10 molecules e 930 kg BB 1. Zeros that arent needed to hold the decimal point are significant. In a physics textbook the speed of light in a vacuum is given as 2998 10 eq8 eq ms.
7596 Now choose from one o Why. A rectangle measures 8759 cm by 351 mm. -21 x 106 5.
Determine the number of significant figures in the following measurements. The number of significant figures s 5 s_5 s 5. Determine the number of significant figures in each of the following measurements.
Both 0000098 and 098 contain two significant figures. 4070L 1081070 m 2. 1201 mL 352 mL 6 mL Answer.
Determine the number of significant figures in each of the following measurements.

Free Homework Practice With Significant Figure Counting And Calculations Persuasive Writing Prompts Chemistry Classroom Chemistry Worksheets

Significant Figures Teaching Chemistry Chemistry Classroom College Chemistry

Making Measurements Reading Scales On Lab Equipment Notes Practice Problems In 2022 Reference Letter Solar System Information Graduated Cylinders

Determine The Number Of Sig Figs In A Measurement Physical Science Fig Math

Significant Figures Rules Teaching Chemistry Chemistry Classroom Chemistry Lessons

Significant Digits Figures Jeopardy Game Powerpoint Presentation This Review Game Has 6 Categories With 5 Review Games Teaching Chemistry Teaching Biology

Table With Rules For Significant Figures In Measured Numbers School Hacks Math Algebra

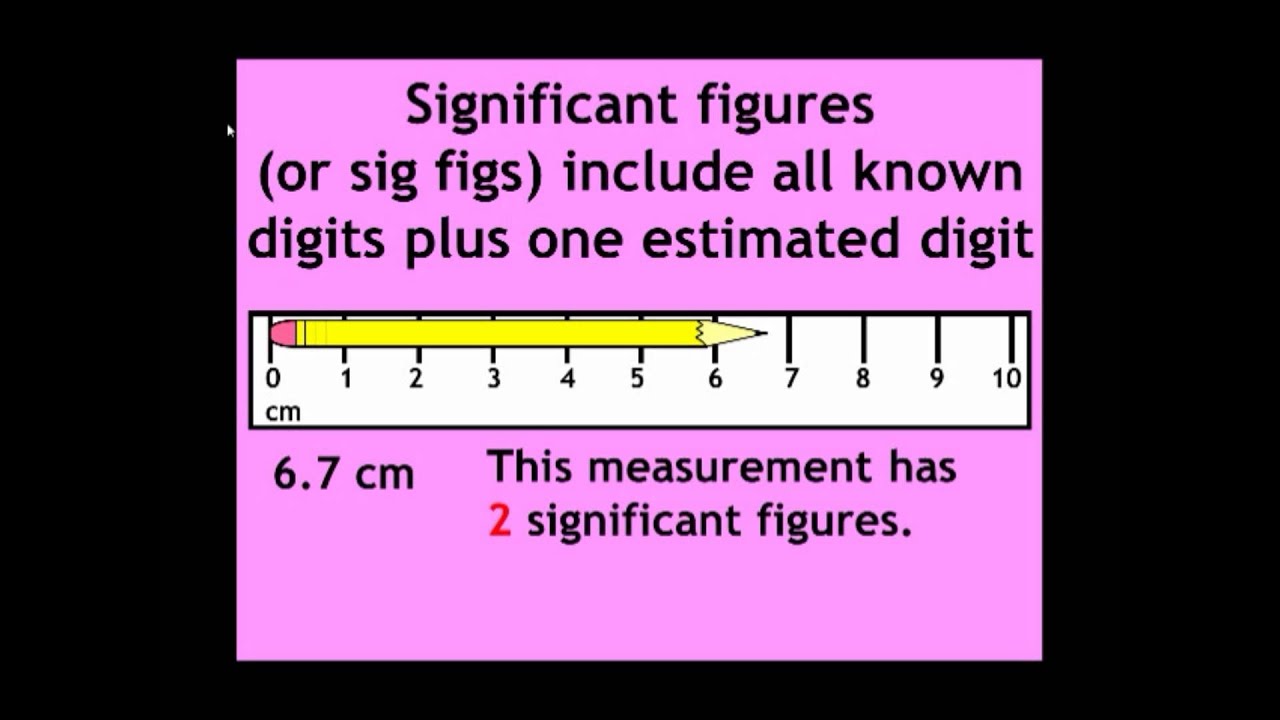
Significant Figures Part I Video Lesson Lesson Video Lessons Student Work

Significant Figures Rules Counting Significant Figures Practice In 2022 Notes Template Reference Letter Compound Words

Significant Figures Rules Counting Significant Figures Practice In 2022 Compound Words Reference Letter Student Learning

Significant Figures Passy S World Of Mathematics Teaching Chemistry Teaching Mathematics

Significant Figures With Rulers Graduated Cylinders And More Chemistry Lessons Graduated Cylinders Teaching Chemistry

Significant Figures Worksheet Or Quiz Rules Of Rounding And Calculations Teaching Math Science Student Science Education

Introduction To Significant Figures Worksheet Teaching Chemistry Math Examples Persuasive Writing Prompts

Counting Significant Figures Scientific Notation Chemistry Notations

Significant Figures Rules Counting Significant Figures Practice In 2022 Compound Words Reference Letter Solar System Information



Comments
Post a Comment